

Motion Equity partners acquires majority stake in Banook Group alongside Turenne Sante and its management team

ABL awarded seven-year NIH contract for continued support in HIV prevention products
© Gary Landsman
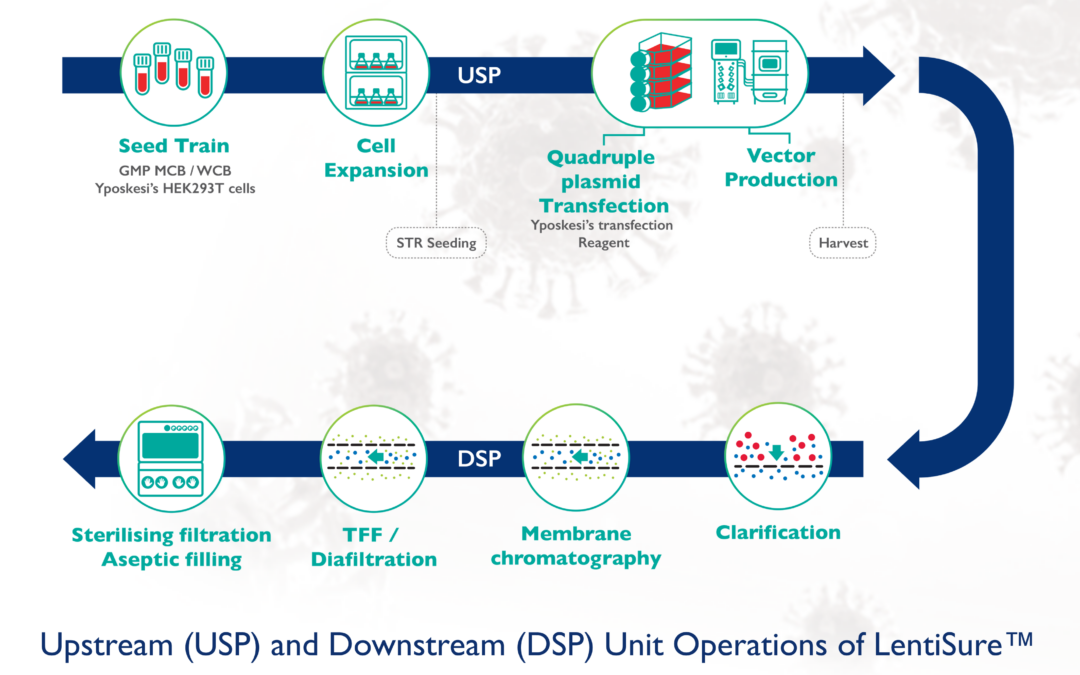
Yposkesi launches LentiSure™, Lentiviral (LV) Vector production platform optimized for higher yields

Labor Dr. Brunner adopts QuantaMatrix dRAST solution in routine clinical use

PDC*line Pharma presents first immunological results from phase I/II trial with PDC*lung01 at ESMO-IO 2022

33 Californie launches CaLySeed, new investment fund providing financial and strategic support to healthcare start-ups

ABL and RD-Biotech sign strategic partnership in cell and gene therapy GMP manufacturing

admed laboratory adopts QuantaMatrix dRAST solution in routine clinical use

