Share this post:
First drinkable formulation of temozolomide for treatment of relapsed or refractory neuroblastoma
Paris and Lyon, France, October 24, 2023 – Orphelia Pharma, a pharmaceutical company dedicated to the development and marketing of pediatric and orphan medicines, today announces the filing of a centralized Marketing Authorization Application (MAA) to the European Medicines Agency (EMA) for KIZFIZO®, the first oral liquid formulation of temozolomide.
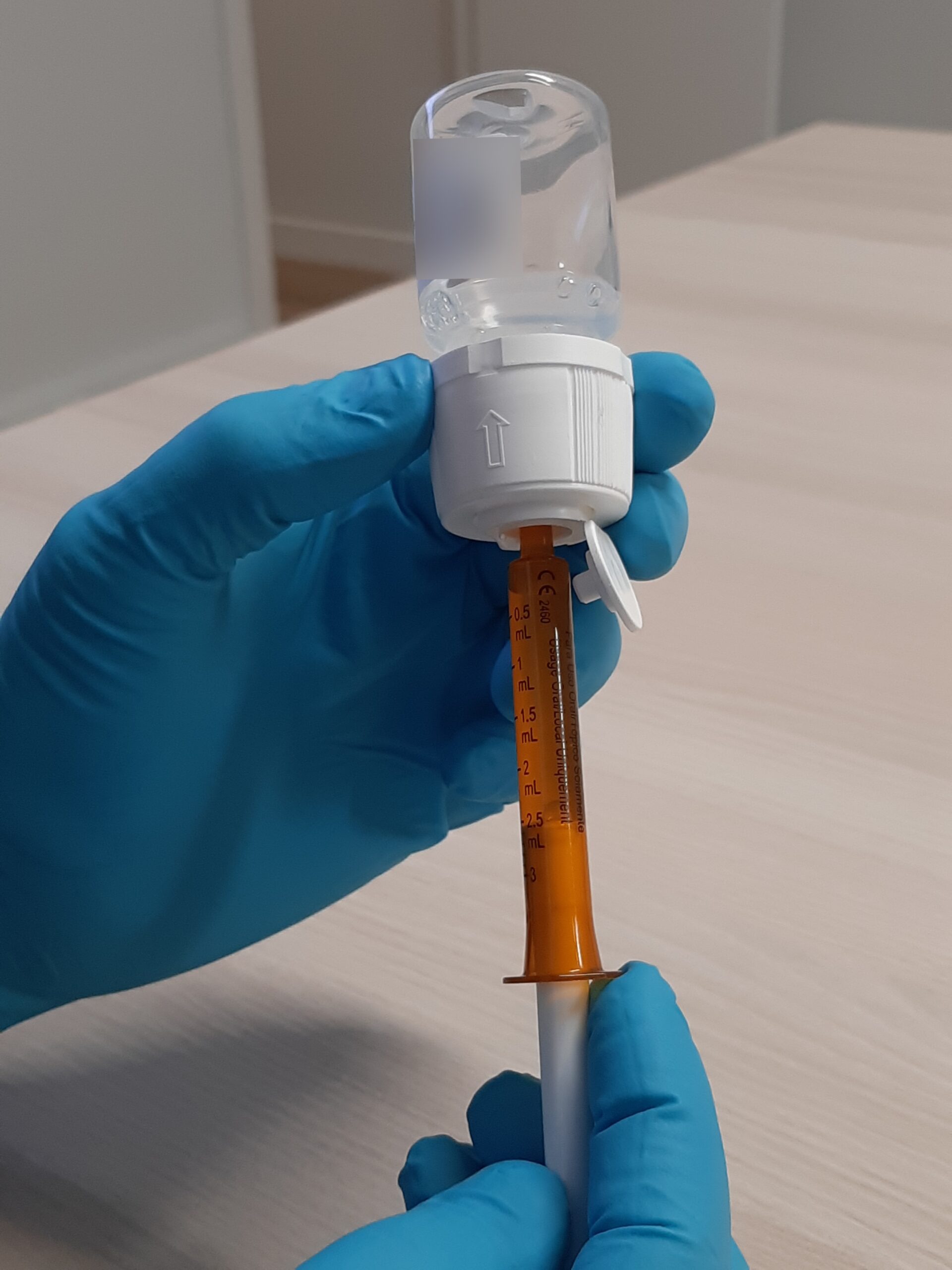
KIZFIZO (temozolomide oral suspension, 40 mg/ml), known as Ped-TMZ or KIMOZO during its clinical development and ongoing early access programs, is designed specifically for use in the treatment of children with relapsed or refractory neuroblastoma, oncology indications with a very poor prognosis. This oral suspension, which is taste-masked, was developed for children: it allows a precise dose to be administered orally or via a nasogastric tube in a small volume. Orphelia Pharma has been developing KIZFIZO in collaboration with Gustave Roussy, the leading European cancer center, for the last six years.
“We take great pride in having developed this new pediatric medicine,” said Laurent Martin, chief pharmaceutical affairs officer at Orphelia Pharma. “KIZFIZO fills an unmet medical need for a drinkable temozolomide medication in the treatment of relapsed or refractory neuroblastoma. This formulation aims to avoid the use of non-age-appropriate dosage forms mixed with a drink or food, which may expose caregivers to a cytotoxic molecule without full control of the dose actually delivered to the patient.”
“The application for a drug marketing authorization of KIZFIZO is excellent news for the children living with cancer and their families. It underscores Gustave Roussy’s commitment to playing a leading role in the development of medicines for children and curing all of them in the future,” said Prof. Fabrice Barlesi, CEO of Gustave Roussy.
The pharmacokinetics of KIZFIZO in children have been evaluated in TEMOkids, a European multicenter population pharmacokinetic acceptability and safety study in pediatric patients in need of temozolomide (NCT04610736).
Efficacy and safety data for temozolomide in relapsed or refractory neuroblastoma submitted in the application includes in particular:
- BEACON-Chemo, a sub-analysis of the chemotherapy arms of the BEACON study, a prospective randomized phase II study in refractory or relapsed neuroblastoma. This study was sponsored by Birmingham University (UK)
- Retro-TMZ, a multicenter descriptive, retrospective study, assessing the efficacy and tolerability of temozolomide in children with refractory or relapsed neuroblastoma. This study was conducted by Gustave Roussy (France)
About KIZFIZO® 40 mg/ml
KIZFIZO (temozolomide oral suspension, 40 mg/ml) is a ready-to-use oral liquid pediatric formulation of temozolomide developed for use in the treatment of relapsed or refractory neuroblastoma, which carry a very poor prognosis. This age-adapted and taste-masked formulation delivers an accurate dose in a small volume, while avoiding drug handling and caregiver exposure to temozolomide. It is the result of a collaboration between the pharmacists and clinicians at Gustave Roussy hospital and the development team at Orphelia Pharma.
In March 2022, KIZFIZO was granted Early Access Authorization (Autorisation d’Accès Précoce) by the French authorities, for the treatment of refractory and relapsed neuroblastoma as monotherapy or in combination with irinotecan or topotecan.
KIZFIZO has received Orphan Drug Designation (ODD) from the EMA and the FDA, the formulation is covered by granted patents and pending applications in Europe and the US.
About neuroblastoma
Neuroblastoma is the most common extracranial cancer in early childhood, with approximately 900 new cases diagnosed per year in the European Union. It almost exclusively affects children under five, with a median age at diagnosis of 18 months. Neuroblastoma has a wide diversity of clinical outcomes, which is reflected in the risk stratification. Approximately 40% of patients have the high-risk disease and often face a poor response to first line induction therapy or later relapse. There remains a high unmet need for relapsed or refractory neuroblastoma patients and the best therapeutic strategy is still an intensive area of research. Temozolomide is the standard chemotherapy and is therefore an essential part of the treatment armamentarium for these patients.
About Orphelia Pharma
Orphelia Pharma is a pharmaceutical company based in Paris and Lyon that develops and markets medicines for the treatment of pediatric and orphan diseases. The company’s mission is to provide patients with essential products in the fields of neurology and oncology, in formulations adapted to the pediatric population. Orphelia Pharma conducts research projects through academic and industrial partnerships. It has recently established regional agreements in European territories and is expanding its footprint across the world.
About Gustave Roussy
Ranked as the leading European Cancer Center and third in the world, Gustave Roussy is a center with comprehensive expertise and is devoted entirely to patients suffering from cancer. The Institute is a founding member of the Paris Saclay Cancer Cluster. It is a source of diagnostic and therapeutic advances. Per year, it caters for almost 50,000 patients, including 3,500 pediatric patients, and its approach is one that integrates research, patient care and teaching. It is specialized in the treatment of rare cancers and complex tumors and it treats all cancers in patients of any age. Its care is personalized and combines the most advanced medical methods with an appreciation of the patient’s human requirements. In addition to the quality of treatment offered, the physical, psychological and social aspects of the patient’s life are respected. 4,100 professionals work on its two campuses: Villejuif and Chevilly-Larue. Gustave Roussy brings together the skills which are essential for the highest quality research in oncology: 40% of patients treated are included in clinical studies.
For further information: www.gustaveroussy.fr/en, Twitter, Facebook, LinkedIn, Instagram
To download documents, you can right-click on the links above and chose « Save link as… »