Share this post:
Company Contact:
Name: Provepharm Inc.
Phone Number: 610-601-8600
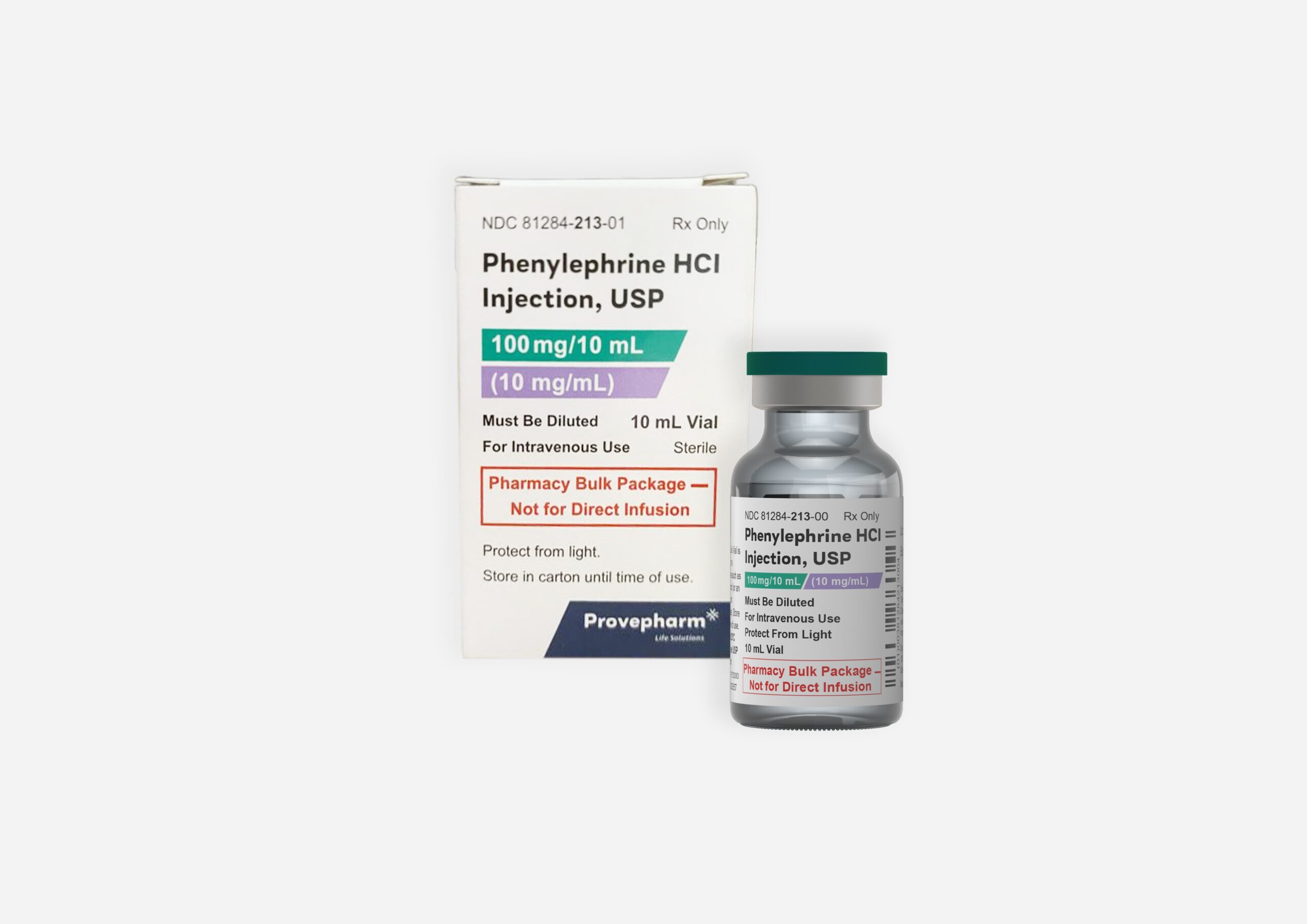
January 24, 2025 – Collegeville, Pennsylvania – Provepharm Inc. is voluntarily recalling lot number 24020027; Expiry Date December 2025 of Phenylephrine hydrochloride Injection, USP, 10 mg/ mL (Pharmacy Bulk Package) at the hospital/institutional level. This recall was initiated based on a customer complaint from a pharmacy after observing a visible black particulate matter found in a single-sealed vial of the product.
Risk Statement:
Administration of an injectable product containing particulate matter may cause local irritation or swelling as a response to the foreign material. If the particulate matter enters the blood vessels, it can travel to various organs and potentially blocking blood vessels in the heart, lungs or brain, leading to serious complications such as stroke or even death. To date, Provepharm Inc. has not received any reports of adverse events or injuries associated with this recall.
Phenylephrine hydrochloride Injection is used for the treatment of clinically important hypotension resulting primarily from vasodilation in the setting of anesthesia and is packaged in 10 mL vial, 1 single dose vial, with NDC code as 81284-213-01.
The product can be identified by product name on carton and vial label and with lot number 24020027 and Exp. Date: Dec 2025 (NDC 81284-213-01) (See enclosed vial label).
Phenylephrine hydrochloride Injection, USP, 10 mg/ mL, was distributed nationwide in the United States to wholesalers.
Provepharm Inc., in collaboration with its recall provider, Sedgwick, is notifying distributors and customers via UPS Ground and coordinating the return of all recalled products.
Wholesalers, distributors, compounding companies and hospitals in procession of the recalled Phenylephrine hydrochloride Injection, USP, 10 mg/ mL lot number 24020027, Exp date December 2025, should immediately cease use of and return the product to Sedgwick at the following address:
- Sedgwick
Event## 8664
2670 Executive Drive, Suite A
Indianapolis, IN 46241
Customers with questions regarding this recall can contact from 8:00 am to 5:00 pm (EST) Monday – Friday at:
- Product Returns:
Contact Sedgwick at:
IVR: 866-737-5394
FAX: 866-250-4503
Email: provepharm8664@sedgwick.com
- Medical-related Questions
Contact Medical Information at:
1-833-727-6556
Email: safety-us@provepharm.com
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online:
www.fda.gov/medwatch/report.htm
- Regular Mail or Fax:
Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
To download documents, you can right-click on the links above and chose « Save link as… »




