Share this post:
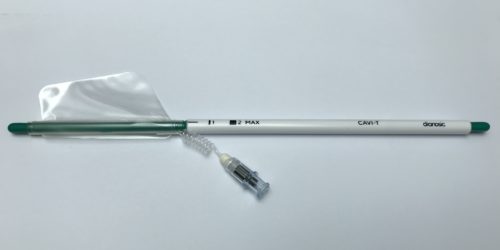
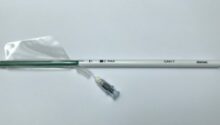
Strasbourg, France, March 10, 2020 – Dianosic, a Strasbourg-based startup specializing in innovative solutions for ear, nose, and throat (ENT) conditions, today announces that it has obtained CE marking for CAVI-TTM, a low-pressure asymmetric balloon for treating spontaneous and post-operative intranasal bleeding. This class IIa certification, obtained at the end of February, will enable the company to begin marketing the device in Europe.
CAVI-T, which is designed to treat intranasal bleeding (epistaxis), is patented in France and will be soon in Europe, the United States, Japan and China. It is the first asymmetric intranasal balloon that stops bleeding by applying low-force compression while adapting to the anatomy of the nasal fossa. The balloon can remain in place for one to three days.
At present, this atraumatic, easy-to-use medical device is aimed primarily at hospitals. A pilot study between April and June 2019, led by Professor Christian Debry, Head of the ENT department at Strasbourg University Hospital, demonstrated its safety and efficacy. The balloon was effective in stopping bleeding in 90% of patients and significantly improved their quality of life. This feasibility study also demonstrated the good safety profile of CAVI-T, with a mean pain score on product insertion and removal of 4.7 and 1.6 respectively. (Evaluated on a Visual Analogue Scale ranging from 1 to 10).
“Obtaining CE marking is an important recognition of Dianosic’s expertise and the ability of its team to conceive and develop innovative products to treat ENT disorders,” said Marc Augustin, President of Dianosic.
“Following this major step in our development, we are ready to prepare the commercial launch of the product in Europe, which is expected to take place during the second quarter of 2020. Dianosic’s entry into the market represents a prime opportunity for growth, with the potential to reach close to five million patients across Europe and the United States,” said Philippe Bastide, CEO of Dianosic.
About Dianosic SAS
Dianosic develops innovative solutions for the management of ENT conditions.
Incubated by SEMIA, named ‘incubator of excellence’ for the Grand Est region in January 2018, Dianosic is led by company President Marc Augustin and CEO Philippe Bastide. Professor Christian Debry, Head of the ENT department at Strasbourg University Hospital Center, acts as a scientific advisor to the company.
Dianosic was a winner at i-Lab 2019, an innovation contest run by Bpifrance and the Ministry of Higher Education, Research and Innovation. Dianosic was also selected to take part in a Soft-Landing program in Boston, MA in September 2019. This program was organized by Strasbourg Eurométropole and Innouvo in partnership with Cajuba Finance.
Founded in July 2017 and based in Strasbourg, Dianosic aims to become a European leader in the management of intranasal bleeding and chronic sinusitis with its active, long-term resorbable treatment.