Share this post:
Écouen, France, October 21, 2019 – Vygon Group, a specialist single-use medical devices group, today announces its acquisition of Italian firm Pilot, which specializes in ECG guidance devices. The financial terms of the agreement have not been disclosed.
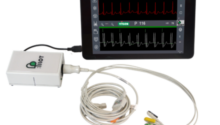
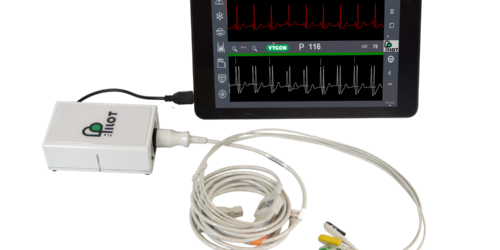
The aim of this transaction is to acquire the ECG location and navigation technology of the Pilottm TLS product, already marketed by Vygon. It is one of only a few devices that enables precise positioning of the distal tip of central venous catheters, including in patients living with atrial fibrillation.
As a result, Vygon will be able to offer its international customers a comprehensive range of intravascular therapies: implantable ports, PICC lines, midlines and central venous catheters, as well as equipment (Vysion XS ultrasound system and ECG Pilot TLS system) to ensure their safe implantation. The new Pilot TLS system offers practitioners a global solution that is particularly appealing when combined with CT PICC easy catheters with unique proximal trimming, as well as with implantable ports. To connect central venous catheters to the Pilot TLS, Vygon’s product offering includes a Vygocard ECG connector using a saline solution for signal transmission. These products will be available in Vygon’s international markets.
Pilot TLS manufacturing and its future development will be handled within the Vygon group at its production and development site in Medwin, near Montpelier, France, which specializes in active medical devices.
The Pilot TLS ECG guidance system is an alternative technique to radioscopy and fluoroscopy, ensuring safe and precise insertion of central venous catheters by confirming the correct positioning of their distal tip at the entrance of the heart. The technique thus reduces the risks associated with repeated exposure to X-rays for patients, particularly children, as well as for healthcare professionals inserting the catheters. Pilot TLS is the only device currently available on the market with a specific mode for patients living with atrial fibrillation. This condition affects around 10% of the elderly population and up to 28% of cancer patients, depending on the type of cancer. Pilot TLS is one of the only ECG endovascular guidance systems certified for use on all patients, ranging from neonatal to adults.
“This new acquisition is part of our group’s external growth strategy,” said Stéphane Regnault, CEO of Vygon. “We have been working with Pilot for the last three years. The internalization of its ECG guidance system will enable us to consolidate our offering of intravascular therapies on the international market.”
About Vygon Group
Vygon designs, manufactures and markets high-tech single-use medical devices for healthcare professionals in hospital and for private and independent practitioners. Vygon is a world leader within this industry, offering a wide range of products in a number of clinical specialties. Organized in five business units (Intensive Care – Neonatology, Enteral & Obstetrics – Intravascular Therapies – Cardiovascular & Surgery – Anesthesia & Emergency), Vygon combines local and international in-depth expertise and know-how in each individual field. With expertise right along the value chain, from product design to the delivery of training for medical personnel, Vygon provides health care professionals with effective and innovative products tailored to their needs and those of their patients, for optimum use and safety. The company distributes over 205 million products a year in more than 120 countries through its network of 26 subsidiaries and 331 distributors. Vygon products display the CE and/or FDA mark and are manufactured in the group’s eight factories in Europe, the USA and Colombia. A family company founded in 1962, Vygon is based in Ecouen, in France’s Greater Paris region. It is a mid-sized business employing 2,350 staff worldwide. The turnover in 2018 was €323 million ($365M), with 81% of this derived from Vygon’s international business.