Share this post:
Compliance with international quality management standards for medical devices kicks off Ubiplug’s entry into vascular access market
Caen, France, March 29, 2022 – Ubiplug, a designer and developer of easy-to-use medical devices to enhance the healthcare of patients undergoing vascular access procedures, today announces it has obtained ISO 13485:2016 certification for its Quality Management System (QMS), which was issued by DNV – Accredia.
Compliance with international regulatory and normative requirements for medical devices allows Ubiplug to begin marketing, to global markets, its patented smart valve system that makes hemodialysis procedures easier. Ubiplug acquired the certification in less than a year, marking the first of four major milestones the company wishes to achieve in 2022.
Ubiplug’s smart valve system for vascular access procedures is based on a fluid management multichannel connection technology. The company’s first application is in hemodialysis, a complex treatment performed several times per week to enable people to live with the chronic kidney disorder: End-Stage Renal Disease (ESRD). There are more than 1.4m ESRD patients in the US and Europe. “ISO 13485:2016 certification for Ubiplug’s QMS is a significant achievement. The compliance with normative and regulatory requirements ensures ‘Safety and Performance’ of our medical devices. We are proud to offer such quality to patients and healthcare professionals, who are our main focus,” said Eric Jean, CEO of Ubiplug. “This certification also gives us the green light on several company activities. We are in position to start our first in-human clinical investigation, followed by the launch of our smart valve system.”
Ubiplug’s smart valve system for improved healthcare is the result of a collaboration with the University Hospital CHU Caen, AP-HP (Europe’s largest public hospital system located in Paris) and Air Liquide Healthcare. The commercial launch of Ubiplug’s first product is earmarked for Q3, 2022.
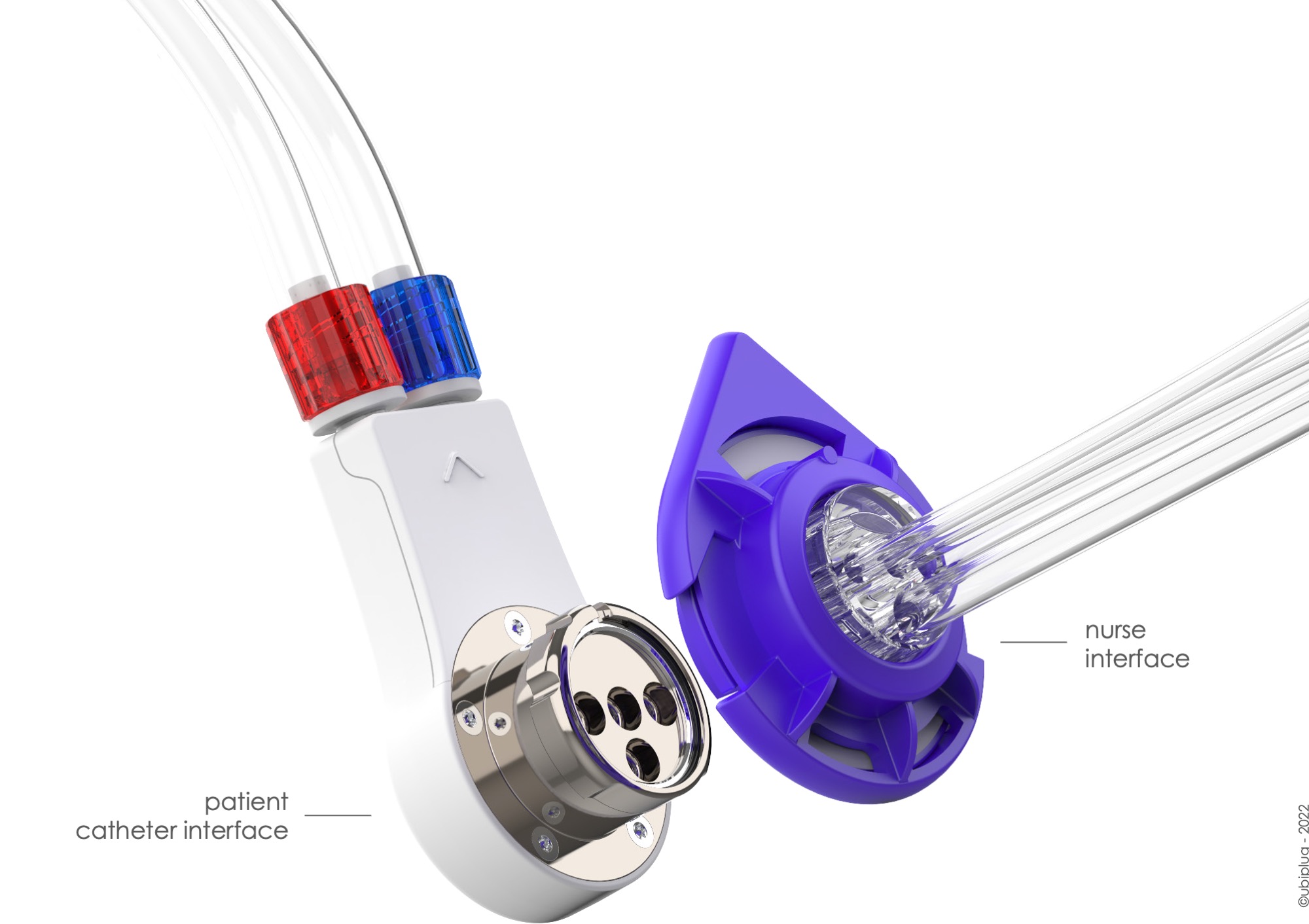
How Ubiplug’s smart valve system works
Ubiplug’s smart valve system is a single-connection device that manages all the necessary steps required to carry out a standard Central Venous Catheter (CVC) procedure. In practice, this means that a nurse directly handles the hemodialysis catheter only once, as opposed to the dozen hand maneuvers usually made. Thanks to Ubiplug’s proprietary innovative technology – covered by five patents and CE marking certified – almost all these individual hand maneuvers are eliminated as the nurse can now switch hemodialysis session steps without having to open the hemodialysis catheter. Revolutionary in its design, Ubiplug’s smart valve system offers the all-important advantage of minimizing patient exposure to the main sources of potentially life-threatening infections.
Note to editor:
ISO 13485 is designed to be used by organizations involved in the design, production, installation and servicing of medical devices and related services. Ubiplug obtained certification within the following scope:
- Design and development of non-active and non-implantable components, semi-finished components and medical devices for use during hemodialysis procedures
- Management of manufacturing of components and semi-finished components for non-active and non-implantable medical devices for use during hemodialysis procedures
- Distribution of non-active and non-implantable sterile medical devices for use during hemodialysis procedures
About Ubiplug
Ubiplug designs and develops easy-to-use medical devices to enhance the healthcare of patients undergoing vascular access. Hemodialysis is the first application of the company’s proprietary technology, Hemoplug™, a platform aimed at transforming how health practitioners care for end-stage renal patients. Ubiplug’s new smart valve system makes it easier to perform vascular access. This single-connection device manages all the necessary steps required to carry out a standard Central Venous Catheter (CVC) procedure, offering the advantage of minimizing patient exposure to the main sources of potentially life-threatening infections.
Founded in 2017 in Caen, France, Ubiplug has raised more than €4M ($4.5M). It collaborates with the University Hospital CHU Caen, AP-HP (Europe’s largest public hospital system located in Paris) and Air Liquide Healthcare, among other industrial and hospital partners, in conceptualizing valve system-based innovations for improved healthcare. The company employs 15 staff.
To download documents, you can right-click on the links above and chose « Save link as… »