Share this post:
Belgian medtech company sponsors comparative trial in France, on use of endomina® in endoscopic gastroplasty procedures to treat patients with type II diabetes and class I obesity
Important step in technique gaining reimbursement approval from France’s national health authority
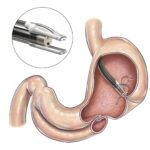
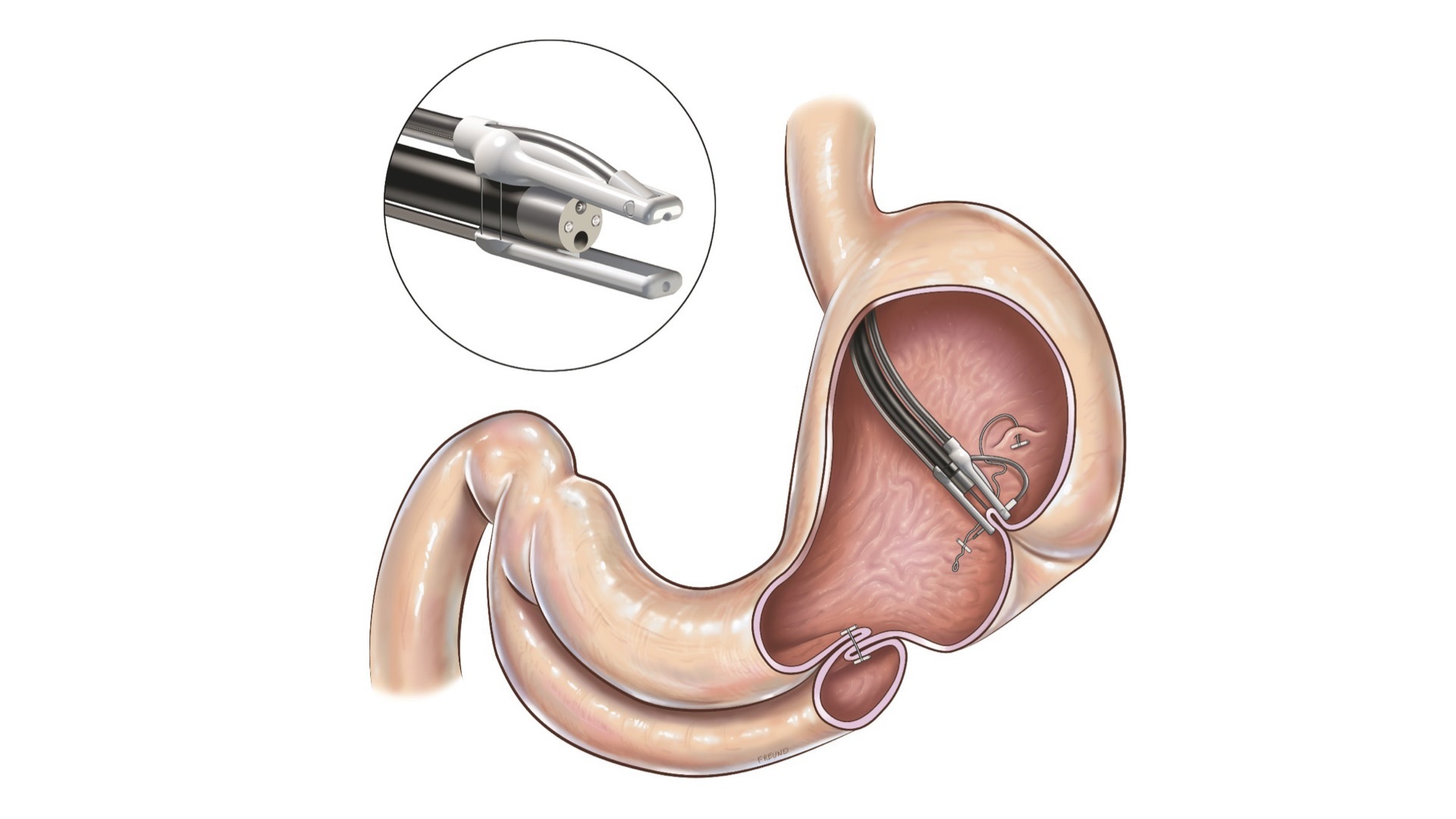
Gosselies, Belgium, October 25, 2023 – Endo Tools Therapeutics (ETT), a company that develops and markets advanced endoscopic medical devices in Europe and the US, today announces the enrolment of the first patient in its ESTIME clinical trial. The trial focuses on ETT’s endomina® platform, which enables endoscopists to perform a volume reduction of the stomach using a minimally invasive procedure performed through the patient’s mouth.
The aim of this prospective, multicenter randomized controlled trial is to demonstrate that an endoscopic gastroplasty performed with the endomina triangulation platform and the TAPES suturing unit can help type II diabetes patients enter remission through weight loss or reduce their use of antidiabetic medications. The trial will also evaluate whether the procedure improves patients’ quality of life. It will comprise 11 trial sites in France and 205 study participants. The procedure has the potential to transform the treatment options available to patients with type II diabetes and class I obesity.
The first patient was recruited onto the trial, led by endocrinologist Professor Anne-Laure Borel, on September 29, 2023, at Grenoble University Hospital. Following approval by France’s national health authority (HAS – Haute Autorité de Santé) in June 2022, the procedure will be subsidized by the Assurance Maladie insurance fund at a select group of healthcare establishments in France, as part of its Forfait Innovation special funding program. This is the first study of its kind to be funded by a Forfait Innovation.
“This clinical trial provides a unique opportunity for patients with type II diabetes and class I obesity to receive new treatments. It is essential that we evaluate innovative weight-loss treatment solutions, so that we can reduce the effects of type II diabetes, a major comorbidity of obesity. If this trial shows the treatment to be effective, it could be a very interesting option for patients,” said Prof. Borel.
“We are delighted that the minister for health has granted early support and special funding for the ESTIME trial, allowing patients to be treated within this study. The data from the trial will be critical in gaining reimbursement for this technique, which will add a new treatment option for patients with type II diabetes and obesity,” said Alexandre Chau, CEO of Endo Tools Therapeutics.
According to the International Diabetes Federation (IDF), around 537 million adults around the world are living with diabetes. In 2021, the condition caused 6.7 million deaths worldwide. Type II diabetes is the most common type of diabetes and is closely linked to obesity; of the three million people in France with type II diabetes, 41% (1.2 million people) are obese. This figure is estimated to be 23 million in Europe and 18 million in the US.
The clinical benefits of Endomina on obesity have already been proven in routine clinical practice. These findings support the results from earlier clinical trials, with an expected average excess weight loss of over 45% at 12 months.
About Endo Tools Therapeutics
Located in Gosselies, Belgium, Endo Tools Therapeutics S.A. (ETT) offers a portfolio of advanced, incisionless and minimally invasive endoscopic medical devices. The company’s mission is to develop solutions that expand the range of gastrointestinal procedures that can be performed using standard gastroscopes, such as bariatric surgery, Endoscopic Full-Thickness Resection (EFTR) and GastroEsophageal Reflux (GER). By developing minimally invasive treatment solutions, ETT aims to reduce the number of complications and the length of hospital stay for patients, and in doing so increase their comfort. The initial results from bariatric procedures performed using ETT’s soft tissue approximation devices were published in the following journals: GIE in 2017, Endoscopy in 2018, Gut in 2020 and EIO in 2022.
To date, over 900 procedures have been performed using Endo Tools Therapeutics’ devices across Europe, the US and the Middle East.
In Europe, the devices are approved for use in endoscopic gastroplasty procedures for the treatment of obesity, while in the US they are cleared for tissue apposition.
To download documents, you can right-click on the links above and chose « Save link as… »