Share this post:
Results from second CID (Cilio-scleral Interposition Device) study confirm sustained decrease in intraocular pressure (IOP), while preserving anterior chamber of eye
Clinical data on CID, the first in a new category of glaucoma implants, will be shared during World Glaucoma Congress in Rome, June 28 to July 2, 2023
Chavanod (near Annecy), France, June 28, 2023 – Ciliatech, an ophthalmology medtech company developing a new class of implant to treat glaucoma durably, today announces it will present the preliminary results of the second two-year post-operative clinical trial (SAFARI II) on its Cilia-scleral Interposition Device (CID) during the World Glaucoma Congress, taking place this week.
Ciliatech will present results showing stable intraocular pressure (IOP) over time, within a safe zone average of 15 mmHg, among its second cohort of patients with Primary Open-Angle Glaucoma (POAG) who underwent a new cilio-scleral surgical procedure.
The findings will show the majority of patients no longer requiring glaucoma prescription medicine (-88%) and no treatment failure. Observations in patients showed that CID is safe. There were no new adverse events reported since the 12-month follow-up visit.
“The results of this second study (SAFARI II) are extremely important in confirming the performance and safety profile in all aspects of our CID implant. These findings are consistent with the IOP management outcomes of our first 24-month post-operative study (SAFARI I),” said Olivier Benoit, CEO of Ciliatech. ”The statistics are stronger now, indicating that our results are not by chance. Ciliatech is proud to have been selected to present the results of our second 24-month post-operative study on CID at the WGC in Rome.”
SAFARI II: two-year post-operative clinical study
The trial was carried out in Yerevan, Armenia, under the supervision of principal investigator Dr. Lilit Voskanyan, ophthalmologist, MD, PhD and head of the Glaucoma Department in Ophthalmology at the Malayan Center. A baseline group of 21 patients enrolled in the SAFARI II study in April 2021; among these, 11 participated in the 24-month follow-up.
The patients in the study had Primary Open-Angle Glaucoma (POAG), non-controlled by glaucoma medication; none had previously had a glaucoma surgery. They each received two samples of a first-generation CID implant as a standalone procedure, using the cilio-scleral surgical technique. Patients were then prescribed a prostaglandins treatment for a maximum of one month. IOP, medications and safety parameters were controlled during several follow-up visits by the principal investigator. None of the patients needed additional incisional therapy, laser or other adjunctive treatments.
Ciliatech views the SAFARI II results obtained as clinically meaningful, with 95% pf patients below 21 mmHg (81% below 18 mmHg), and 90% free of medication at 24 months.
“On top of the very good safety profile, we are particularly satisfied with the high number of patients free of medication at 24 months, adding to the positive prognosis for prolonged effect. This significantly improves patient quality of life, which is the expectation following surgery,” said Dr. Philippe Sourdille, co-founder of Ciliatech and inventor of CID.
At WGC, Ciliatech will present its results in the exhibition poster area, poster nb 842-P.
Next steps
Currently, Ciliatech has more than 100 patients enrolled in various clinical studies. Its SAFARI I and II studies combined cover the data of 27 patients at two years. To date, one surgeon has conducted all of its studies. The company’s aim is to have its preliminary results confirmed by other surgeons and in other populations, such as narrow angle glaucoma for which its CID implant – using a cilio-scleral ab externo procedure – has the potential to treat.
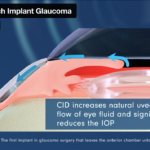
CID is a groundbreaking device disrupting traditional approaches in glaucoma surgery. It is the first implant that enables surgeons to prolong reduced IOP while bypassing the anterior of the eye and sparing the conjunctiva.
A CID procedure uses the eye’s natural capacity to drain ocular aqueous humor from the anterior chamber into physiological channels. This cilio-scleral approach means that a whole host of post-operative complications are resolved. Notably, there is no filtration bleb and no ECL loss. There is no ‘loss of chance’ should a patient require a second surgery. This approach enables this second surgery to be performed without negative consequences on its efficacy and safety.
About Ciliatech
Ciliatech, a medtech company specialized in ophthalmology, is developing a new class of implant to address the increasing need to treat glaucoma durably and with zero adverse effects. Glaucoma affects 80M people per year worldwide. The company’s groundbreaking concept CID (Cilio-scleral Inter-positioning Device) is the first implant in the industry to reduce intraocular pressure (IOP) without penetrating the anterior chamber or creating subconjunctival filtration – critical criteria that overcome the most serious complications and shortcomings of other glaucoma surgical techniques. As CID is embedded between only two areas of the eye (uniquely between the ciliary body and the sclera), it offers the unparalleled advantage of unlocking the natural uveoscleral pathway without altering the anterior segment of the eye.
Founded in 2017 by ophthalmic surgeon and inventor Philippe Sourdille, and Olivier Benoit, a veteran engineer and biotech entrepreneur, Ciliatech recently launched a third clinical trial to test the latest generation of its implant. The company is located near Annecy, France, and to date has raised €6M ($6.5M) in financing.
www.cilia.tech
To download documents, you can right-click on the links above and chose « Save link as… »