Share this post:
- Preliminary 12-month results show excellent performance and safety profile
- CID glaucoma implant works similarly in both open and narrow angle glaucoma patients, without the need to remove lens
- 86% of patients remain medication-free
Ophthalmology Futures Symposium, Vienna, Austria, September 7, 2023 – Ciliatech, an ophthalmology medtech company developing a new class of implant to treat glaucoma durably, today announces it will present at the Ophthalmology Futures Symposium the preliminary results of a one-year study of its clinical trial, SAFARI III, involving both open and narrow angle glaucoma patients.
Preliminary outcomes show an excellent performance and safety profile, with very few, mild, adverse events reported. Results indicate that Ciliatech’s glaucoma implant CID (Cilioscleral Interpositioning Device) works similarly for patients with Primary Open Angle Glaucoma (POAG), the most common type of glaucoma worldwide, and Primary Narrow Angle Glaucoma, also called Primary Angle Closure Glaucoma (PACG), without the need to remove the lens. This is a significant benefit in Ciliatech’s cilioscleral approach.
A cohort of 57 patients participated in the SAFARI III clinical trial, with similar numbers of open and narrow angle patients. At the 12-month post-operative visit, intraocular pressure (IOP) was reduced by more than 41% (23.5 mmHg at baseline down to 13.9 mmHg at M12). Pharmacological treatments were reduced by more than 92% (mean medication dropped from 1.7 at baseline to 0.1 at M12). At 12 months, 86% of patients reported being medication-free.
“The preliminary SAFARI III results are exciting. In addition to avoiding Endothelial Cells Loss, CID is the first minimally invasive surgical device that shows substantial promise in benefitting both open and narrow angle glaucoma patients. This is a paradigm shift for narrow angle patients care. They will now have the possibility of postponing more invasive and old-fashioned incisional therapies and can take advantage of the performance and the safety that CID offers in treating their glaucoma,” said Olivier Benoit, CEO of Ciliatech.
Cilioscleral: a promising approach for treating open and narrow angle glaucoma
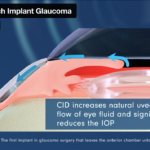
The cilioscleral approach, unique in leaving the anterior chamber of the eye untouched, also avoids removing the lens, which currently, Ciliatech says, is regularly performed in patients with narrow angle glaucoma, even if it is ‘not always justified’ by a cataractous lens. The SAFARI III study outcomes tend to show that removing the lens is unnecessary.
Glaucoma is a chronic optic neuropathy, characterized by atrophy of the optic nerve and loss of retinal ganglion cells, resulting in progressive vision loss in most cases due to sight threatening high IOP. Chronic glaucoma is divided into ‘open’ angle and ‘narrow’ angle. The angle is formed by the iris and the cornea. When this angle is more than 20 degrees, it is ‘Open’, and if lower it is ‘Narrow’.
The prevalence rates of PACG patients are estimated at 15 to 20% of European and US populations, but more than 50% in patients of Asian descent. Patients with ACG have a two-fold greater risk of vision loss during their lifetime.
While progress has been made in treating glaucoma with lower adverse effects using minimally invasive implants (MIGS) and improving standards of care for POAG patients, these types of implants are usually incompatible for PACG patients.
“PACG is a real challenge that is underserved by the implants available today. Ciliatech’s CID and cilioscleral surgical approach are angle agnostic, so that the angle of the glaucoma does not matter in performing the surgery. OAG and ACG patients can now be treated equally, with the same level of safety and performance, as indicated in our preliminary 12-month SAFARI III outcomes. The CID is the first minimally invasive implant to benefit PACG patients,” added Olivier Benoit.
SAFARI III study
Dr. Lilit Voskanyan, ophthalmologist, MD, PhD and head of the glaucoma department in ophthalmology at the Malayan Center in Yerevan, Armenia, operated on 57 patients from June to December 2022. The study cohort was composed of two sub-groups of equal proportions of POAG and PACG patients.
Patients enrolled had glaucoma with IOP > 21mmHg, uncontrolled by medications. Operations were performed as a stand-alone procedure (i.e., not combined with cataract). Patients were seen at baseline, with follow-up visits for safety and performance control scheduled at regular intervals.
Ophthalmology futures symposium presentation:
- ‘CID and cilioscleral surgical approach clinical outcomes – 12-month follow-up results on PACG patients’
- Speaker: Olivier Benoit, CEO of Ciliatech
- Thursday, September 7, at 09:30 – Hilton Danube Waterfront Hotel
About Ciliatech
Ciliatech, a medtech company specialized in ophthalmology, is developing a new class of implant to address the increasing need to treat glaucoma durably and with zero adverse effects. Glaucoma affects 80M people per year worldwide. The company’s groundbreaking concept CID (Cilioscleral Interpositioning Device) is the first implant in the industry to reduce intraocular pressure (IOP) without penetrating the anterior chamber or creating subconjunctival filtration – critical criteria that overcome the most serious complications and shortcomings of other glaucoma surgical techniques. As CID is embedded between only two areas of the eye (uniquely between the ciliary body and the sclera), it offers the unparalleled advantage of unlocking the natural uveoscleral pathway without altering the anterior segment of the eye.
Founded in 2017 by ophthalmic surgeon and inventor Philippe Sourdille, and Olivier Benoit, a veteran engineer and biotech entrepreneur, Ciliatech recently launched a fourth clinical trial on a second-generation implant. The company is located near Annecy, France, and to date has raised €6M ($6.5M) in financing.
To download documents, you can right-click on the links above and chose « Save link as… »