Share this post:
Results presented at Ophthalmology Futures European Forum will show:
- Sustained IOP (intraocular pressure) control of 8.0mmHg/33% reduction vs baseline
- 70% reduction in medication dependance among patients
- 68% of patients remain medication-free at three years, with no significant safety reports
Ophthalmology Futures Forums, Barcelona, Spain, September 5, 2024 – Ciliatech, an innovative medtech company focusing on the treatment of glaucoma, today announces it will present the combined results of a 36-month follow-up of its SAFARI I & II clinical trials on its groundbreaking Intercil® Uveal Spacer, a Cilioscleral Interpositioning Device (CID), at the Ophthalmology Futures European Forum, taking place today.
The Ophthalmology Futures European Forum brings together clinicians, scientists, regulators and business leaders to discuss the latest developments and trends in ophthalmology.
Ciliatech will present three-year efficacy and safety data on 41 patients with Primary Open-Angle Glaucoma (POAG) who underwent a CID surgical procedure. Results will show enduring IOP (intraocular pressure) control of 8.0mmHg with a mean pressure reduction of 33% compared to baseline and with a 70% reduction in medication dependance. Over the 36 months, the majority of patients (68%) remained medication free. There have been no new safety reports since the 24-month follow up visits.
Ciliatech will also affirm that there has been no need for additional glaucoma surgeries for any patients treated across the SAFARI studies.
“Similar to any new approach, we are very focused on assuring efficacy and safety. Ciliatech is excited and proud to present longer-term data on its novel CID implant, Intercil Uveal Spacer. These latest results showing a sustained decrease in IOP, in conjunction with a remarkable IOP-lowering medication decrease, confirm the CID concept, whereby the IOP is controlled without the need to open the anterior chamber of the eye. It is also important to note that the safety profile is excellent and that no patient has had to undergo another IOP-lowering procedure during the follow-up period,” said Olivier Benoit, CEO of Ciliatech.
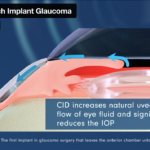
It is well established within scientific literature that the uveoscleral outflow pathway is compromised with age and disease. Ciliatech’s Intercil Uveal Spacer seeks to uniquely re-establish this natural outflow pathway without needing to enter the anterior chamber of the eye.
“Our novel approach demonstrates an unprecedented level of efficacy, with an equally reassuring safety profile. Going forward, these clinical results can be presented to regulatory approval bodies, surgeons and patients alike to provide assurance and drive uptake of the device on the market,” added Benoit.
Ciliatech plans to continue following-up on current studies, beyond this existing three-year time point, as well as embark upon additional multi-center studies around the world towards further scientific validation of its Intercil Uveal Spacer.
Ophthalmology futures symposium presentation:
- ‘Cilioscleral Interposition technique and CID – long term clinical data’
- Speaker: Olivier Benoit, CEO of Ciliatech
- Thursday, September 5, at 08:40 – The Esferic event hall, Barcelona
About SAFARI I and II
Ciliatech’s clinical trial study program seeks to validate the hypothesis that SuprAciliary Filtration Alone Reduces IOP (SAFARI). Its SAFARI I and II studies are Ciliatech’s first-in-human trials designed to demonstrate the safety and efficacy of CID SV13 (a first-generation Intercil Uveal Spacer) in patients with POAG. Combined, the studies cover the data of 41 patients at three years. A simple Ab-Externo surgical implantation technique is utilized to place the Intercil Uveal Spacers sub-sclerally without ever entering the anterior chamber of the eye. This is performed in a straightforward standalone procedure. Various efficacy and safety parameters are then assessed over long-term study visits, along with associated Ultrasound Bio Microscopy (UBM) imaging to further validate safe placement of the devices.
About Ciliatech
Ciliatech is an innovative medtech company focusing on the treatment of glaucoma – a disease that affects 80 million people worldwide. Its Intercil® Uveal Spacer, a groundbreaking implant in the new ‘Cilioscleral interpositioning Device’ (CID) category, will be the first proprietary glaucoma device that treats both the open and narrow angle forms of the disease. Clinical trial follow-up of patients implanted with the device has now reached the three-year mark; demonstrating highly positive results in robust IOP lowering coupled with a very high safety profile. Multiple clinical trials of the second-generation CID are in progress, whilst the company is actively pursuing regulatory approvals. CID’s bespoke surgical implantation technique – facilitating external placement into the supraciliary space of the eye without ever entering the eye’s anterior chamber – sets it apart from other glaucoma procedures.
Co-founded in 2017 by ophthalmic surgeon and inventor Dr. Philippe Sourdille, and Olivier Benoit, a veteran engineer and biotech entrepreneur, Ciliatech has to date raised €6m ($6.5m) and is expanding its scientific, medical and commercial team. The company is located near Annecy, France.
Legal disclaimer: Ciliatech products are currently not available for sale pending regulatory approvals.
To download documents, you can right-click on the links above and chose « Save link as… »