Share this post:
Article published in Alzheimer’s Research & Therapy describes results of a pilot clinical trial conducted at AP-HP
First results demonstrate safety of transient repeated BBB opening with SonoCloud in Alzheimer’s patients, and show potential of technology as a new therapeutic approach for treatment of Alzheimer’s disease
Paris, France, March 29, 2022 – Carthera, a French company that designs and develops SonoCloud, an innovative ultrasound-based medical device to treat a wide range of brain diseases, announces today the publication of results in the Alzheimer’s Research & Therapy Journal, from an investigator-sponsored trial evaluating the safety and efficacy of the use of the SonoCloud technology in patients with mild Alzheimer’s Disease (AD).
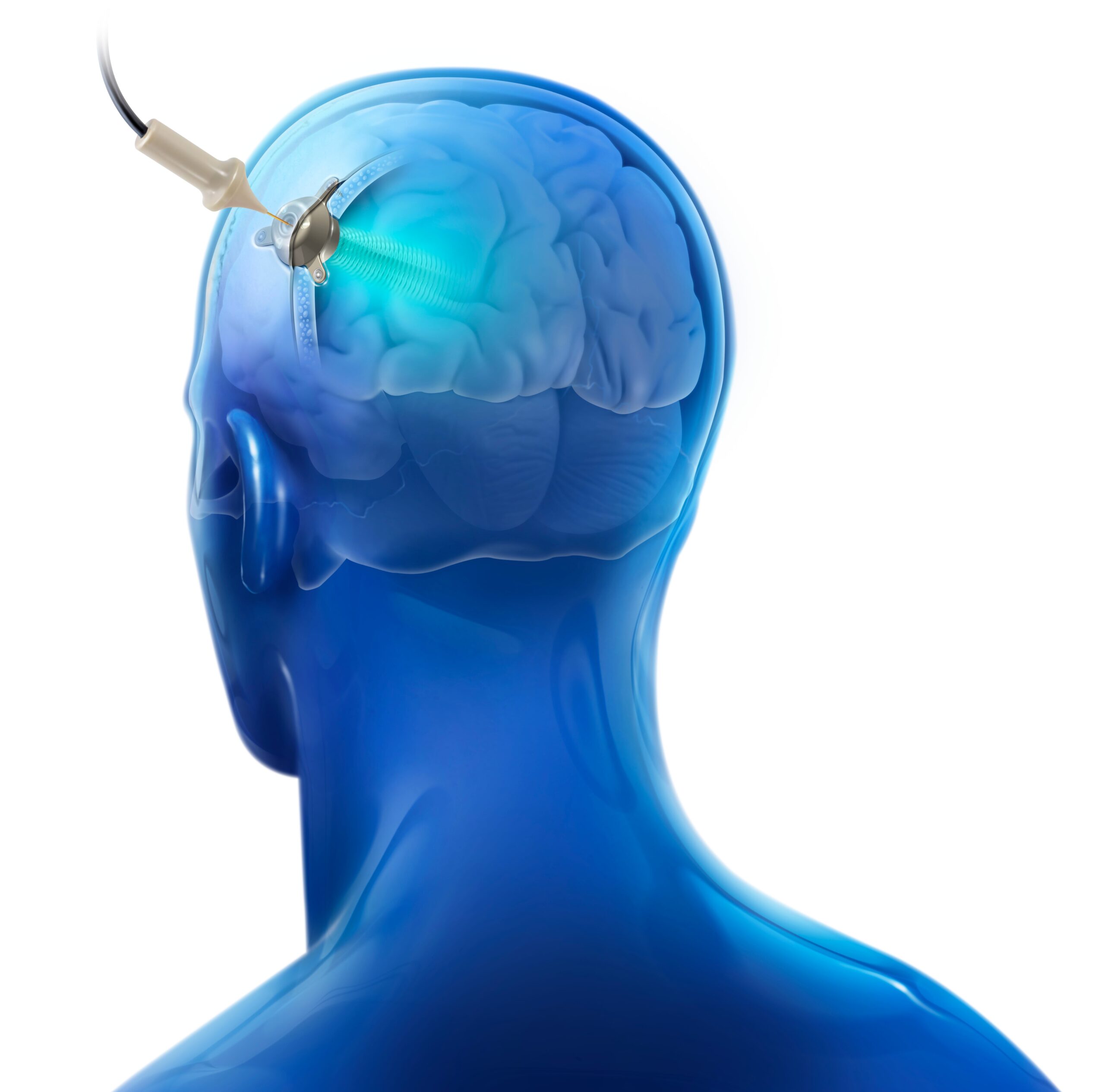
In the past decade, the use of Low-Intensity Pulsed Ultrasound (LIPU) has been shown to temporarily disrupt the Blood-Brain Barrier (BBB), reduce β-amyloid and tau pathologies, and improve cognitive performance in Alzheimer’s preclinical models. SonoCloud, an implantable 1 MHz ultrasound device that can be activated on demand using a transdermal needle connected to an external interface, harnesses the therapeutic potential of this technique in an easy-to-use system that allows for repeated treatments in patients.
A translational study was conducted at the Hôpital de la Pitié-Salpêtrière (AP-HP Sorbonne, Paris, France) by Pr. Alexandre Carpentier and Dr. Stéphane Epelbaum. It follows previous trials that showed the potential of SonoCloud to safely enhance the delivery of chemotherapy to patients with brain tumors. The goal of this study was to demonstrate the safety of this technique in patients with mild AD and to explore whether ultrasound alone could reduce their amyloid load.
A single-emitter version of the SonoCloud was implanted, under local anesthesia, in patients with mild AD to target the left supramarginal gyrus. Over three and a half months, seven ultrasound sessions (lasting ~ten minutes each) were performed twice a month on nine patients to temporarily disrupt the BBB. PET imaging was performed at inclusion, and at four and eight months after the initial sonications, to monitor brain metabolism and amyloid levels.
The trial, conducted by ‘Assistance Publique Hopitaux de Paris (AP-HP)’ showed that the SonoCloud can disrupt the BBB in Alzheimer’s patients and further confirmed the safety previously demonstrated in patients with brain tumors, published in 2019 in Clinical Cancer Research. Furthermore, a slight decrease in amyloid load was reported in the majority of patients despite the short treatment and observation window, confirming the therapeutic potential of this modality, as has been observed in preclinical models.
“The study’s findings complement the promising results already published and confirm the significant role that the SonoCloud device can play in the treatment of a wide spectrum of brain diseases, particularly if coupled with a novel drug therapy. Carthera is actively seeking collaborations with pharma partners to further develop this technique and allow a greater number of patients to benefit from this innovative treatment,” said Pr. Alexandre Carpentier.
“We’re currently planning a clinical trial that will lead to marketing authorization of the SonoCloud for the treatment of glioblastoma while also continuing to explore this technology in a greater number of brain indications in combination with various therapeutic agents,” added Frédéric Sottilini, CEO of Carthera. “The outcomes of this study reinforce our conviction that the SonoCloud has the potential to unlock the efficacy of therapies for brain diseases that were previously untreatable.”
About AP-HP
Europe’s leading Hospital and University Center (CHU), the AP-HP, and its 39 hospitals are organized into six university hospital groups (AP-HP Centre – University of Paris; AP-HP. Sorbonne University; AP-HP Nord – University of Paris; AP-HP University of Paris Saclay; AP-HP Henri Mondor and AP-HP University Hospitals; University Hospitals Paris Seine-Saint-Denis) and structured around five Paris universities. Closely linked to major research organizations, AP-HP has four world-class university hospital institutes (ICM, ICAN, IMAGINE, FOReSIGHT) and the largest French health data warehouse (EDS). A major player in applied research and health innovation, the AP-HP holds a portfolio of 650 active patents, its clinician researchers sign nearly 10,000 scientific publications each year and more than 4,000 research projects are currently under development, all sponsors combined. In 2015, the AP-HP also created the AP-HP Research Foundation to support biomedical and health research conducted in all its hospitals.
www.aphp.fr
About Carthera
Carthera is a clinical-stage medtech company focused on developing innovative ultrasound-based medical devices to treat a wide range of brain disorders.
The company is a spin-off from AP-HP Paris and Sorbonne University. Carthera leverages the inventions of Pr. Alexandre Carpentier, head neurosurgeon at AP-HP Sorbonne university, who has achieved worldwide recognition for his innovative developments in treating brain disorders. Carthera is developing the SonoCloud, an intracranial implant that temporarily opens the Blood-Brain Barrier (BBB). The device is currently in clinical trials in Europe and in the United States.
Founded in 2010, Carthera has offices in France (Lyon and Paris) and a subsidiary in the United States. Since its inception, the technical and clinical development of the SonoCloud has received support from the National Research Agency (ANR), the French public investment bank (Bpifrance), the National Institutes of Health (NIH) and the European Innovation Council (EIC).
www.carthera.eu
To download documents, you can right-click on the links above and chose « Save link as… »




