Share this post:
Paris, France, May 10, 2017 – CarThera, a French company that designs and develops innovative ultrasound-based medical devices to treat brain disorders, today announces that Dr. Ahmed Idbaih, AP-HP principal investigator of the Phase 1/2a clinical trial (NCT02253212) on ultrasound induced Blood-Brain Barrier opening will reveal preliminary safety and efficacy data at the 2017 American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago (June 2-6). The clinical trial involved 21 patients with recurrent glioblastoma (GBM), who have all been treated with SonoCloud® low-intensity pulse ultrasound in a total of 65 sessions.
The presentation will take place at the McCormick Place Convention Center in Chicago, Illinois on June 5, during the poster session on Central Nervous System Tumors (1:15pm-4:45pm CDT). The abstract #2034 titled ‘Phase 1/2a study of an implantable device delivering low intensity pulsed ultrasound (LIPU) to disrupt the blood-brain barrier (BBB), followed by intravenous carboplatin chemotherapy in patients with recurrent GBM‘ will be released on abstracts.asco.org by ASCO on May 17, 2017, at 5:00pm CDT.
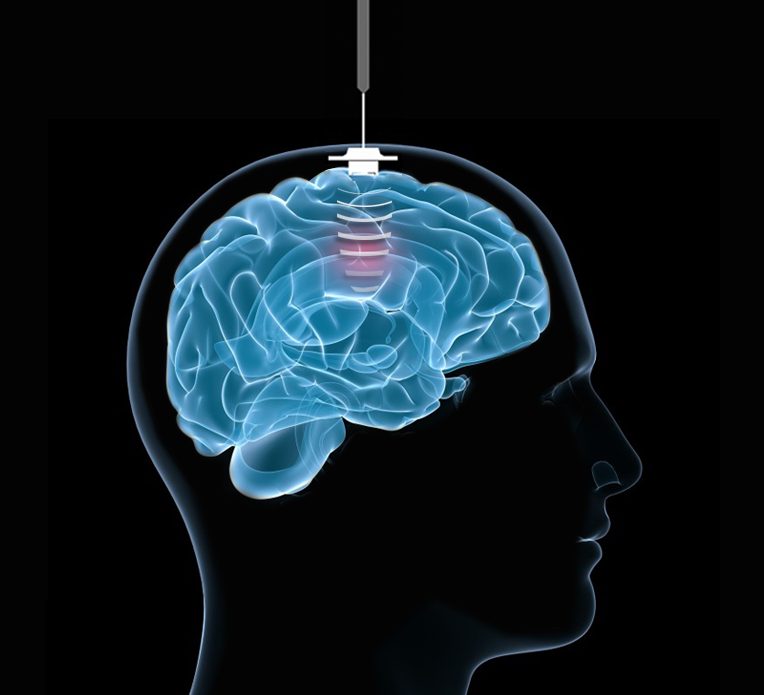
The BBB prevents the passage of most drugs from the blood to the brain and may be responsible for the limited efficacy of current chemotherapies in GBM patients. To tackle this problem, Pr. Alexandre Carpentier, French neurosurgeon at AP-HP and founder of CarThera, developed a low-intensity pulse ultrasound device called SonoCloud.
SonoCloud allows the blood vessels in the brain to become temporarily permeable, improving delivery of intravenous therapeutic molecules, including chemotherapy drugs.
In June 2016, CarThera announced the preliminary data of its Phase 1/2a clinical trial with 15 relapsing glioblastoma patients. A detailed article was published in Science Translational Medicine (http://stm.sciencemag.org/content/8/343/343re2), showing that the device’s tolerability and safety profile were good. This was achieved in association with teams from the Assistance Publique-Hôpitaux de Paris (the Greater Paris University Hospitals) and the Pierre and Marie Curie University.
“The BBB is one of the key challenges in neuroscience and neuro-oncology. We are delighted and honored that Dr. Idbaih’s abstract has been selected from among the 5,700 submitted and that the SonoCloud data can be presented at the prestigious ASCO Annual Meeting,” said Frederic Sottilini, CEO of CarThera. “We believe that low-intensity pulsed ultrasound (LIPU) will become the new leading approach for treating patients with brain cancer and, potentially, neurodegenerative indications.”
“We are excited to be able to share the preliminary data from our clinical trial at the ASCO Annual Meeting with more than 30,000 oncology professionals from around the world,” said Dr. Idbaih, principal investigator for the clinical trial and neuro-oncologist at Pitié-Salpêtrière Hospital. “We are delighted to share our experience of BBB opening for the treatment of glioblastoma at recurrence with the medical community at this important event.”
According to the company’s estimates, each year 250,000 patients worldwide are diagnosed with a brain tumor. More than 160,000 of them could benefit from the SonoCloud breakthrough, mainly those with primary brain cancers and some brain metastases of other cancers. This represents a market worth €1.5 billion ($1.7bn).
About SonoCloud
SonoCloud® is an innovative medical device developed by CarThera. It is capable of emitting ultrasound to temporarily increase the permeability of the blood vessels in the brain, with the aim of increasing the delivery of therapeutic molecules. Created by Professor Alexandre Carpentier and developed in collaboration with the Laboratory of Therapeutic Applications of Ultrasound (Laboratoire Thérapie et Applications Ultrasonores, LabTAU) at INSERM, SonoCloud is an implant inserted into the skull after a regular surgical procedure and activated prior to each round of chemotherapy. Two minutes of low intensity ultrasound emission is enough to open the blood brain barrier for six hours and to increase by five to seven times the concentration of therapeutic molecules delivered into the brain, with good tolerance. This ultrasound-induced disruption of the blood-brain barrier is a world first; it offers a new treatment option for a wide range of indications, including Alzheimer’s disease.
About CarThera
CarThera designs and develops innovative therapeutic ultrasound-based medical devices for treating brain disorders. The company is a spin-off from AP-HP, Greater Paris University Hospitals, the largest hospital group in Europe, and Pierre and Marie Curie University (UPMC). CarThera leverages the inventions of Professor Alexandre Carpentier, a neurosurgeon at AP-HP who has achieved worldwide recognition for his innovative developments in treating brain disorders. CarThera developed SonoCloud, an intracranial ultrasound implant that temporarily opens the bloodbrain barrier (BBB). Founded in 2010 by Professor Alexandre Carpentier, CarThera is based at the Brain and Spine Institute (Institut du Cerveau et de la Moelle épinière, ICM) in Paris, France, and has laboratories at the Bioparc Laënnec business incubator in Lyon, France. The company, led by Frederic Sottilini (CEO), works closely with the Laboratory of Therapeutic Applications of Ultrasound (Laboratoire Thérapie et Applications Ultrasonores, LabTAU, INSERM) in Lyon. Since its inception, the company has received support from France’s Ministry of Research, the Ile-de-France region, the Bpifrance public investment bank, Medicen Paris Region and Lyonbiopôle.
To download documents, you can right-click on the links above and chose « Save link as… »




