Share this post:
Paris, France, April 2, 2019 – CarThera, a French company that designs and develops innovative ultrasound-based medical devices to treat brain disorders, today announces the publication in AACR’s Clinical Cancer Research journal of the final results of its Phase I/IIa clinical trial (NCT02253212) on the ultrasound-induced blood-brain barrier (BBB) opening improving the efficiency of carboplatin chemotherapy.
Study results show a good safety profile for the ultrasound implant, SonoCloud-1. During the study, 19 patients with recurrent glioblastoma (GBM) received up to ten monthly ultrasound sonications.
These results are based on 65 sonication sessions with escalating ultrasound pressure levels. GBM patients treated with optimal pressure levels and MRI-detected disrupted BBB (11 patients) showed a median progression-free survival (PFS) of 4.11 months and a median overall survival (OS) of 12.94 months. This compares to 2.73 months and 8.64 months respectively, for GBM patients without BBB disruption (8 patients).
The clinical trial took place at the AP-HP Pitie Salpetriere Hospital in Paris, led by Dr. Ahmed Idbaih, principal investigator and neuro-oncologist, together with Professor Alexander Carpentier, neurosurgeon at AP-HP, head of the neurosurgery department at Pitie Salpetriere Hospital and founder of CarThera.
“The results of the trial suggest a potentially greater efficacy for carboplatin when used in combination with the device to disrupt the BBB. We are now looking forward to confirming this in a larger clinical trial,” said Dr. Ahmed Idbaih. “These results reinforce our hypothesis that local, temporary opening of the BBB using low-intensity pulsed ultrasound delivered in a controlled manner enables better chemotherapy penetration.”
“This trial clearly shows the potential of the SonoCloud technology to enhance the brain penetration of both existing and new drugs for GBM and other brain diseases,” said Pr. Alexandre Carpentier. “This is the first trial in the world presenting the feasibility and safety results of iterative BBB opening with significant indications for better tumor control and preserved neurological conditions.”
“We are thrilled with the results of our first clinical study using the SonoCloud-1implant,” said Frederic Sottilini, CEO of CarThera. “We are now very keen on moving forward with our next trials using our new advanced device, the SonoCloud-9; enhancing the penetration of chemotherapy in a larger volume in order to further improve survival rates for GBM patients.”
CarThera is now recruiting recurrent GBM patients for its new clinical trial (NCT03744026) with the SonoCloud-9 implantable ultrasound device, which is designed to cover a volume that is nine times larger than the previous generation device. This advanced device will cover the tumor and surrounding infiltrative regions.
About Gliobastoma
Glioblastoma is the most frequent and most aggressive type of brain tumor. It can occur at any age, but 70% of cases are diagnosed in patients aged between 45 and 70. With an incidence in North America and Europe of 2 to 5 cases per 100,000 people, it accounts for more than 50% of primary malignant brain tumors. There are an estimated 250,000 new cases of glioblastoma worldwide every year. It is one of the most aggressive types of brain tumor, with a poor prognosis. Throughout the world, annually there are around 200,000 deaths from glioblastoma, including nearly 15,000 in Europe and 9,000 in the US. Current treatment consists of surgery to remove as much of the tumor as possible followed by a combination of radiotherapy and temozolomide chemotherapy. Patients with tumor recurrence can have repeat surgery and are offered second-line chemotherapy, although this can be of little benefit.
About SonoCloud
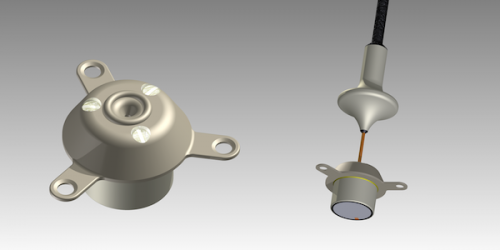
SonoCloud® is an innovative medical device developed by CarThera. It emits ultrasound to temporarily increase the permeability of the blood vessels in the brain. Invented by Pr. Alexandre Carpentier, SonoCloud is an implant inserted into the skull and activated prior to chemotherapy. Several minutes of low-intensity ultrasound opens the blood brain barrier for six hours and increases the concentration of therapeutic molecules in the brain. The SonoCloud technology is appropriate for the treatment of brain diseases in general. Oncology indications are the company’s primary target but investigations are ongoing in other conditions, including neurodegenerative diseases and Alzheimer’s disease in particular.
About CarThera
CarThera designs and develops innovative therapeutic ultrasound-based medical devices for treating brain disorders. The company is a spin-off from AP-HP, Greater Paris University Hospitals, the largest hospital group in Europe, and Sorbonne University. CarThera leverages the inventions of Professor Alexandre Carpentier, a neurosurgeon at AP-HP who has achieved worldwide recognition for his innovative developments in treating brain disorders. CarThera developed SonoCloud, an intracranial ultrasound implant that temporarily opens the blood-brain barrier (BBB).
Founded in 2010 by Professor Alexandre Carpentier, CarThera is based at the Brain and Spine Institute (Institut du Cerveau et de la Moelle épinière, ICM) in Paris, France, and has laboratories at the Bioparc Laënnec business incubator in Lyon, France. The company, led by Frederic Sottilini (CEO), works closely with the Laboratory of Therapeutic Applications of Ultrasound (Laboratoire Thérapie et Applications Ultrasonores, LabTAU, INSERM) in Lyon. Since its inception, the company has received support from the APHP, Sorbonne University, the ANR (Nationale Research Agency), France’s Ministry of Research, the Ile-de-France region, the Bpifrance public investment bank, the Medicen Paris Region and Lyonbiopôle clusters.
http://carthera.eu/ @CarThera_





