


Provepharm receives FDA marketing approval in US for BLUDIGO, its indigo carmine injection drug product
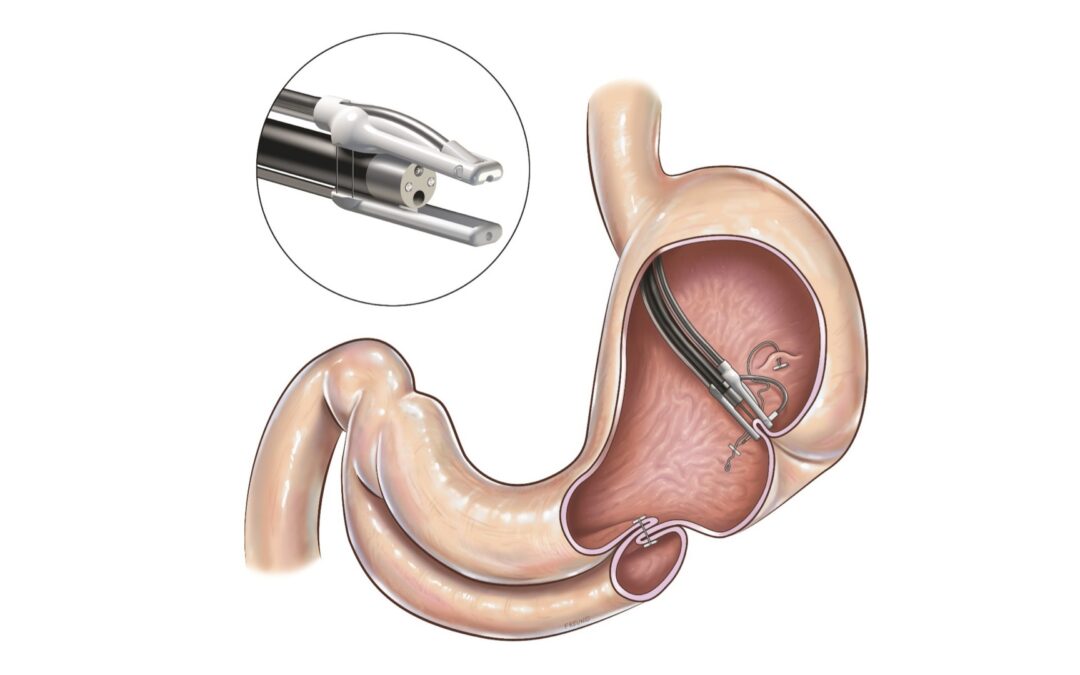
Endo Tools Therapeutics receives positive opinion from French High Authority for Health on Forfait Innovation scheme to treat patients with diabetes

Deciphera obtient l’autorisation de la commission européenne pour le QINLOCK® (RIPRETINIB) en traitement de quatrième ligne des tumeurs stromales gastro-intestinales (GIST)

Amolyt Pharma obtient la désignation de médicament orphelin de la FDA pour l’AZP-3601 pour le traitement potentiel de l’hypoparathyroïdie

Nosopharm obtains US patent for NOSO-502, a first-in-class novel antibiotic
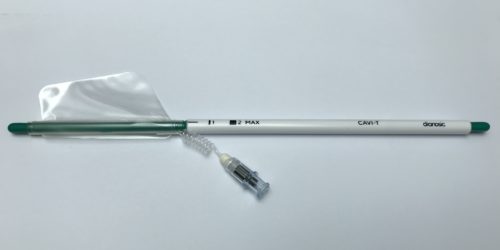
Dianosic obtains CE marking for CAVI-T, medical device for treating intranasal bleeding

Domain Therapeutics is granted European and US patent for biosensors assessing GPCR trafficking

Minoryx Therapeutics receives US FDA fast-track designation for leriglitazone in the treatment of X-ALD

